Benzene
| Infobox on Benzene | |
|---|---|
| Example of Benzene |  |
| Facts | |
| Origin | - |
| Stowage factor (in m3/t) | - |
| Humidity / moisture | - |
| Ventilation | - |
| Risk factors | See text |
Benzene
Description / Application
Benzene (also called benzol – coal naphta - ) is the lightest fraction obtained from the distillation of coal tar hydrocarbons (produced by coking coal in the steel industries) and is synthesised from natural gas. Benzene occurs naturally in Crude Oil. It is a thin, colourless, highly flammable liquid with a characteristic smell. It is a good motor fuel solvent as well as being a feedstock for other benzene derivatives used in dye, insecticides and plastics production. At a later stage in the distillation process, a heavy coal tar solvent naphtha and a naphthalene fraction are produced. The fraction is further treated to yield naphthalene, a white crystalline solid with a penetrating tarry smell. Naphthalene is the most abundant of the aromatic hydrocarbons, being important as the starting point for a number of dyes and also as a source of phthalic acid which in turn leads to indigo and to certain resins used in very large quantities in paint. Naphthalene can be reduced to a series of compounds such as tetralin and decalin which have great value as solvents.
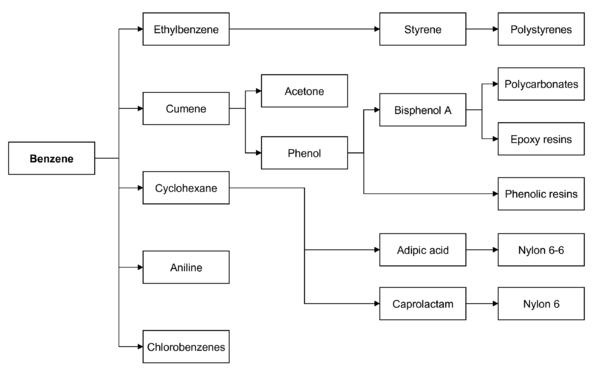
Benzene is by far the most important of the aromatics and its principal derivatives are cyclohexane, cumene, ethyl benzene and alkyl benzene. Cyclohexane accounts for approximately 23% of the benzene usage. Ethyl benzene represents approximately 45% of the benzene production and is used in the manufacture of styrene which in turn is used to produce polystyrene and ABS (acrylonitrile/buradiene/styrene) resins, both of which should enjoy strong growth rates. Cumene is used to make phenol and alkyl benzene goes toward the manufacture of detergents.
Benzene is used mainly as an intermediate to make other chemicals. About 80% of benzene is consumed in the production of three chemicals, ethylbenzene, cumene, and cyclohexane. Its most widely produced derivative is ethylbenzene, precursor to styrene, which is used to make polymers and plastics. Cumene is converted phenol for resins and adhesives. Cyclohexane is used in the manufacture of Nylon. Smaller amounts of benzene are used to make some types of rubbers, lubricants, dyes, detergents, drugs, explosives, and pesticides.
In both the US and Europe, 50% of benzene is used in the production of ethylbenzene/styrene, 20% is used in the production of cumene, and about 15% of benzene is used in the production of cyclohexane (eventually to nylon).
As a gasoline (petrol) additive, benzene increases the octane rating and reduces knocking. As a consequence, gasoline often contained several percent benzene before the 1950s, when tetraethyl lead replaced it as the most widely used antiknock additive. With the global phaseout of leaded gasoline, benzene has made a comeback as a gasoline additive in some nations. In the United States, concern over its negative health effects and the possibility of benzene's entering the groundwater have led to stringent regulation of gasoline's benzene content, with limits typically around 1%. European petrol specifications now contain the same 1% limit on benzene content. The United States Environmental Protection Agency introduced new regulations in 2011 that lowered the benzene content in gasoline to 0.62%.
In laboratory research, toluene is now often used as a substitute for benzene. The solvent-properties of the two are similar, but toluene is less toxic and has a wider liquid range.
Shipment / Storage
Benzene is a colourless to light-yellow, mobile, non-polar liquid of highly refractive nature; aromatic odour; vapours burn with smoky flame.
- Boiling point; 80°C
- Flashpoint; -11°C
- Autoignition temp.; 562°C
Miscible with alcohol, ether, acetone, carbon tetrachloride, carbon disulfide, acetic acid; slightly soluble in water.
For overseas carriage aspects of Chemicals, the readers are recommended to acquire or have access to a good chemical dictionary, and a copy of the International Maritime Dangerous Goods (IMDG) Code, issued by the International Maritime Organisation. Also consult the applicable MSDS sheet.
See also: http://www.chemicalland21.com/petrochemical/BENZENE.htm#
Risk factors
Carcinogen. Highly toxic. Flammable; dangerous fire risk. Explosive limits in air 1,5% to 8% by volume. TLV: 10 ppm in air.
According to the Agency for Toxic Substances and Disease Registry (ATSDR) (2007), benzene is both an anthropogenically produced and naturally occurring chemical from processes that include: volcanic eruptions, wild fires, synthesis of chemicals such as phenol, production of synthetic fibers, and fabrication of rubbers, lubricants, pesticides, medications, and dyes. The major sources of benzene exposure are tobacco smoke, automobile service stations, exhaust from motor vehicles, and industrial emissions; however, ingestion and dermal absorption of benzene can also occur through contact with contaminated water. Benzene is hepatically metabolized and excreted in the urine. Measurement of air and water levels of benzene is accomplished through collection via activated charcoal tubes, which are then analyzed with a gas chromatograph. The measurement of benzene in humans can be accomplished via urine, blood, and breath tests; however, all of these have their limitations because benzene is rapidly metabolized in the human body into by-products called metabolites.
OSHA regulates levels of benzene in the workplace. The maximum allowable amount of benzene in workroom air during an 8-hour workday, 40-hour workweek is 1 ppm. Because benzene can cause cancer, NIOSH recommends that all workers wear special breathing equipment when they are likely to be exposed to benzene at levels exceeding the recommended (8-hour) exposure limit of 0.1 ppm











