Difference between revisions of "Nitrogenous Fertiliser"
(→Description) |
|||
| Line 10: | Line 10: | ||
Nitrogenous [[fertilisers]] are mineral and organic substances used as a source of nitrogen nutrition for [[plants]]. They are divided into organic fertilizers (manure, peat, compost) containing other nutrients besides nitrogen; manufactured mineral fertilizers; and green fertilizers (lupine, serradella).<br><br> | Nitrogenous [[fertilisers]] are mineral and organic substances used as a source of nitrogen nutrition for [[plants]]. They are divided into organic fertilizers (manure, peat, compost) containing other nutrients besides nitrogen; manufactured mineral fertilizers; and green fertilizers (lupine, serradella).<br><br> | ||
<b>Ammonia fertilizers</b> include [[ammonium sulfate]], ammonium chloride, ammonium bicarbonate, and liquid nitrogenous fertilizers. Ammonium sulfate and ammonium chloride are most effective on base-saturated soils (chernozems, calcareous serozems, chestnut) which can neutralize their acidifying effect. Systematic fertilization with ammonium sulfate or ammonium chloride increases soil acidity, which can be corrected by liming. Ammonia nitrogen is less susceptible to leaching than nitrate nitrogen; thus, ammonia fertilizers can be added in the fall, before planting. They are less suitable for surface (as supplementary feeding for winter crops) and local (in rows or by cluster sowing) application. Excessive chlorine in ammonium chloride has an adverse effect on the size and quality of the yield of many crops, including [[potatoes]], [[flax]], oil-producing [[plants]], [[tobacco]], and [[grapes]]. Ammonium bicarbonate, still produced only in limited quantities for experimental purposes, has an alkaline reaction, but it undergoes nitrification in soil. Among the ammonia forms, liquid fertilizers—liquid anhydrous ammonia, aqua ammonia, and ammoniates—are the most important.<br><br> | <b>Ammonia fertilizers</b> include [[ammonium sulfate]], ammonium chloride, ammonium bicarbonate, and liquid nitrogenous fertilizers. Ammonium sulfate and ammonium chloride are most effective on base-saturated soils (chernozems, calcareous serozems, chestnut) which can neutralize their acidifying effect. Systematic fertilization with ammonium sulfate or ammonium chloride increases soil acidity, which can be corrected by liming. Ammonia nitrogen is less susceptible to leaching than nitrate nitrogen; thus, ammonia fertilizers can be added in the fall, before planting. They are less suitable for surface (as supplementary feeding for winter crops) and local (in rows or by cluster sowing) application. Excessive chlorine in ammonium chloride has an adverse effect on the size and quality of the yield of many crops, including [[potatoes]], [[flax]], oil-producing [[plants]], [[tobacco]], and [[grapes]]. Ammonium bicarbonate, still produced only in limited quantities for experimental purposes, has an alkaline reaction, but it undergoes nitrification in soil. Among the ammonia forms, liquid fertilizers—liquid anhydrous ammonia, aqua ammonia, and ammoniates—are the most important.<br><br> | ||
| − | <b>Nitrate fertilizers</b> include sodium nitrate (Chilean saltpeter), calcium nitrate (lime saltpeter, Norwegian saltpeter), and [[ | + | <b>Nitrate fertilizers</b> include sodium nitrate (Chilean saltpeter), calcium nitrate (lime saltpeter, Norwegian saltpeter), and [[Potassium Nitrate]]. Sodium nitrate is physiologically alkaline and is therefore best applied to acid soils, especially when sugar beets, [[wheat]], [[barley]], and other acid-sensitive crops are grown. Calcium nitrate is put up in pellets usually admixed with [[ammonium nitrate]]; it, too, alkalizes the soil. Potassium nitrate contains potassium as well as nitrogen and is a source of nitrogen-potassium nutrition for plants. It is applied to chlorine-sensitive crops. All the nitrate forms of nitrogen are not absorbed by the soil. In regions with excess moisture, nitrate fertilizers are leached out of light soils with weak water-retention capacity. It is best, therefore, to use ammonia fertilizers as the main fertilizers.<br><br> |
<b>Amide fertilizers</b> include [[urea]] ([[carbamide]]), calcium cyanamide, and [[urea]]-[[formaldehyde]]. Urea is the most valuable. In soil it readily changes into ammonium carbonate; it first slightly alkalizes the soil and then weakly acidifies it. It is recommended that urea be added early. It is also used as a protein supplement for ruminants. Calcium cyanamide is able to reduce soil acidity.<br><br> | <b>Amide fertilizers</b> include [[urea]] ([[carbamide]]), calcium cyanamide, and [[urea]]-[[formaldehyde]]. Urea is the most valuable. In soil it readily changes into ammonium carbonate; it first slightly alkalizes the soil and then weakly acidifies it. It is recommended that urea be added early. It is also used as a protein supplement for ruminants. Calcium cyanamide is able to reduce soil acidity.<br><br> | ||
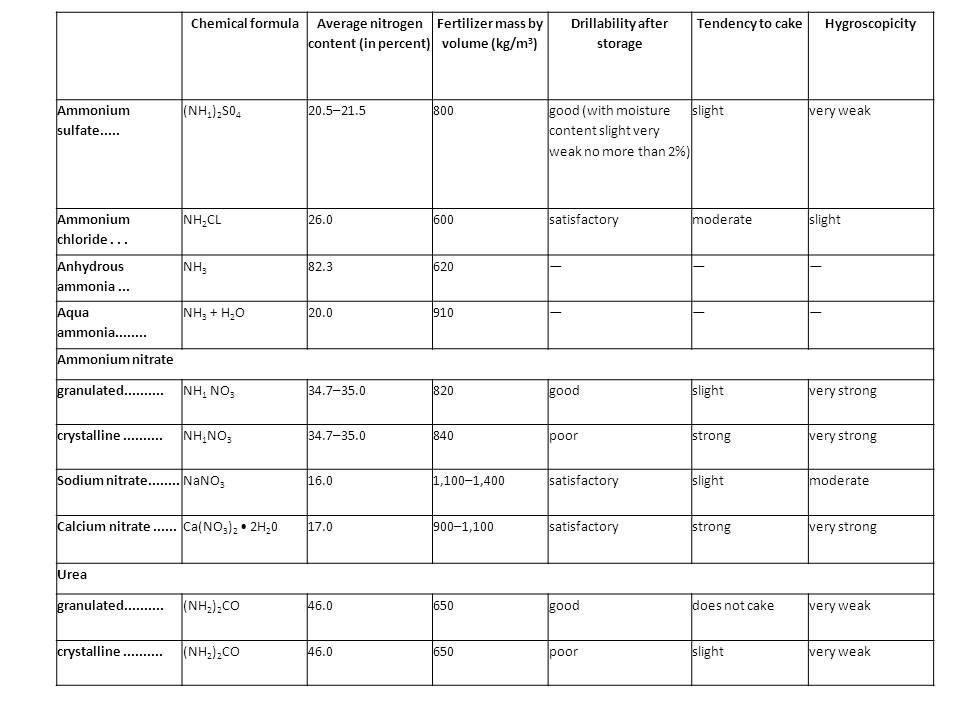
| − | <b>Properties of the main mineral nitrogenous fertilizers | + | <b>Properties of the main mineral nitrogenous fertilizers</b><br> |
[[File:nitrogenus_fertilizers.jpg]] | [[File:nitrogenus_fertilizers.jpg]] | ||
Revision as of 14:36, 29 January 2014
| Infobox on Nitrogenous Fertiliser | |
|---|---|
| Example of Nitrogenous Fertiliser |  |
| Facts | |
| Origin | - |
| Stowage factor (in m3/t) | 1,15 m3/t (in bulk) |
| Humidity / moisture | - |
| Ventilation | - |
| Risk factors | See text |
Nitrogenous Fertiliser
Contents
Description
Nitrogenous fertilisers are mineral and organic substances used as a source of nitrogen nutrition for plants. They are divided into organic fertilizers (manure, peat, compost) containing other nutrients besides nitrogen; manufactured mineral fertilizers; and green fertilizers (lupine, serradella).
Ammonia fertilizers include ammonium sulfate, ammonium chloride, ammonium bicarbonate, and liquid nitrogenous fertilizers. Ammonium sulfate and ammonium chloride are most effective on base-saturated soils (chernozems, calcareous serozems, chestnut) which can neutralize their acidifying effect. Systematic fertilization with ammonium sulfate or ammonium chloride increases soil acidity, which can be corrected by liming. Ammonia nitrogen is less susceptible to leaching than nitrate nitrogen; thus, ammonia fertilizers can be added in the fall, before planting. They are less suitable for surface (as supplementary feeding for winter crops) and local (in rows or by cluster sowing) application. Excessive chlorine in ammonium chloride has an adverse effect on the size and quality of the yield of many crops, including potatoes, flax, oil-producing plants, tobacco, and grapes. Ammonium bicarbonate, still produced only in limited quantities for experimental purposes, has an alkaline reaction, but it undergoes nitrification in soil. Among the ammonia forms, liquid fertilizers—liquid anhydrous ammonia, aqua ammonia, and ammoniates—are the most important.
Nitrate fertilizers include sodium nitrate (Chilean saltpeter), calcium nitrate (lime saltpeter, Norwegian saltpeter), and Potassium Nitrate. Sodium nitrate is physiologically alkaline and is therefore best applied to acid soils, especially when sugar beets, wheat, barley, and other acid-sensitive crops are grown. Calcium nitrate is put up in pellets usually admixed with ammonium nitrate; it, too, alkalizes the soil. Potassium nitrate contains potassium as well as nitrogen and is a source of nitrogen-potassium nutrition for plants. It is applied to chlorine-sensitive crops. All the nitrate forms of nitrogen are not absorbed by the soil. In regions with excess moisture, nitrate fertilizers are leached out of light soils with weak water-retention capacity. It is best, therefore, to use ammonia fertilizers as the main fertilizers.
Amide fertilizers include urea (carbamide), calcium cyanamide, and urea-formaldehyde. Urea is the most valuable. In soil it readily changes into ammonium carbonate; it first slightly alkalizes the soil and then weakly acidifies it. It is recommended that urea be added early. It is also used as a protein supplement for ruminants. Calcium cyanamide is able to reduce soil acidity.
Properties of the main mineral nitrogenous fertilizers