Difference between revisions of "Nitrogenous Fertiliser"
(→Description) |
|||
| (24 intermediate revisions by the same user not shown) | |||
| Line 7: | Line 7: | ||
| risk factors = See text | | risk factors = See text | ||
}} | }} | ||
| + | __TOC__ | ||
==Description== | ==Description== | ||
Nitrogenous [[fertilisers]] are mineral and organic substances used as a source of nitrogen nutrition for [[plants]]. They are divided into organic fertilizers (manure, peat, compost) containing other nutrients besides nitrogen; manufactured mineral fertilizers; and green fertilizers (lupine, serradella).<br><br> | Nitrogenous [[fertilisers]] are mineral and organic substances used as a source of nitrogen nutrition for [[plants]]. They are divided into organic fertilizers (manure, peat, compost) containing other nutrients besides nitrogen; manufactured mineral fertilizers; and green fertilizers (lupine, serradella).<br><br> | ||
<b>Ammonia fertilizers</b> include [[ammonium sulfate]], ammonium chloride, ammonium bicarbonate, and liquid nitrogenous fertilizers. Ammonium sulfate and ammonium chloride are most effective on base-saturated soils (chernozems, calcareous serozems, chestnut) which can neutralize their acidifying effect. Systematic fertilization with ammonium sulfate or ammonium chloride increases soil acidity, which can be corrected by liming. Ammonia nitrogen is less susceptible to leaching than nitrate nitrogen; thus, ammonia fertilizers can be added in the fall, before planting. They are less suitable for surface (as supplementary feeding for winter crops) and local (in rows or by cluster sowing) application. Excessive chlorine in ammonium chloride has an adverse effect on the size and quality of the yield of many crops, including [[potatoes]], [[flax]], oil-producing [[plants]], [[tobacco]], and [[grapes]]. Ammonium bicarbonate, still produced only in limited quantities for experimental purposes, has an alkaline reaction, but it undergoes nitrification in soil. Among the ammonia forms, liquid fertilizers—liquid anhydrous ammonia, aqua ammonia, and ammoniates—are the most important.<br><br> | <b>Ammonia fertilizers</b> include [[ammonium sulfate]], ammonium chloride, ammonium bicarbonate, and liquid nitrogenous fertilizers. Ammonium sulfate and ammonium chloride are most effective on base-saturated soils (chernozems, calcareous serozems, chestnut) which can neutralize their acidifying effect. Systematic fertilization with ammonium sulfate or ammonium chloride increases soil acidity, which can be corrected by liming. Ammonia nitrogen is less susceptible to leaching than nitrate nitrogen; thus, ammonia fertilizers can be added in the fall, before planting. They are less suitable for surface (as supplementary feeding for winter crops) and local (in rows or by cluster sowing) application. Excessive chlorine in ammonium chloride has an adverse effect on the size and quality of the yield of many crops, including [[potatoes]], [[flax]], oil-producing [[plants]], [[tobacco]], and [[grapes]]. Ammonium bicarbonate, still produced only in limited quantities for experimental purposes, has an alkaline reaction, but it undergoes nitrification in soil. Among the ammonia forms, liquid fertilizers—liquid anhydrous ammonia, aqua ammonia, and ammoniates—are the most important.<br><br> | ||
| − | <b>Nitrate fertilizers</b> include sodium nitrate (Chilean saltpeter), calcium nitrate (lime saltpeter, Norwegian saltpeter), and [[Potassium Nitrate]]. Sodium nitrate is physiologically alkaline and is therefore best applied to acid soils, especially when sugar beets, [[wheat]], [[barley]], and other acid-sensitive crops are grown. Calcium nitrate is put up in pellets usually admixed with [[ | + | <b>Nitrate fertilizers</b> include sodium nitrate (Chilean saltpeter), calcium nitrate (lime saltpeter, Norwegian saltpeter), and [[Potassium Nitrate]]. Sodium nitrate is physiologically alkaline and is therefore best applied to acid soils, especially when sugar beets, [[wheat]], [[barley]], and other acid-sensitive crops are grown. Calcium nitrate is put up in pellets usually admixed with [[Ammonium Nitrate]]; it, too, alkalizes the soil. Potassium nitrate contains potassium as well as nitrogen and is a source of nitrogen-potassium nutrition for plants. It is applied to chlorine-sensitive crops. All the nitrate forms of nitrogen are not absorbed by the soil. In regions with excess moisture, nitrate fertilizers are leached out of light soils with weak water-retention capacity. It is best, therefore, to use ammonia fertilizers as the main fertilizers.<br><br> |
<b>Amide fertilizers</b> include [[urea]] ([[carbamide]]), calcium cyanamide, and [[urea]]-[[formaldehyde]]. Urea is the most valuable. In soil it readily changes into ammonium carbonate; it first slightly alkalizes the soil and then weakly acidifies it. It is recommended that urea be added early. It is also used as a protein supplement for ruminants. Calcium cyanamide is able to reduce soil acidity.<br><br> | <b>Amide fertilizers</b> include [[urea]] ([[carbamide]]), calcium cyanamide, and [[urea]]-[[formaldehyde]]. Urea is the most valuable. In soil it readily changes into ammonium carbonate; it first slightly alkalizes the soil and then weakly acidifies it. It is recommended that urea be added early. It is also used as a protein supplement for ruminants. Calcium cyanamide is able to reduce soil acidity.<br><br> | ||
<b>Properties of the main mineral nitrogenous fertilizers</b><br> | <b>Properties of the main mineral nitrogenous fertilizers</b><br> | ||
| − | [[File:nitrogenus_fertilizers.jpg]] | + | [[File:nitrogenus_fertilizers.jpg]]<br><br> |
| + | If applied in the fall, it is effective on mellow neutral soils rich in organic matter. It is unsuitable for local application. Calcium cyanamide is also used as a defoliant to remove leaves from [[cotton]] before it is harvested. Urea-[[formaldehyde]] fertilizers are not leached out of the soil and are especially effective in regions of excess moisture and irrigated agriculture. The use of large amounts provide crops with enough nitrogen to last several years. The characteristics of mineral nitrogenous fertilizers are given in the table.<br><br> | ||
| + | Nitrogenous fertilizers are an effective means of increasing crop productivity, especially in the nonchernozem zone, humid regions of the forest steppe, and the zone of irrigated agriculture where the soils do not contain enough nitrogen. The fertilization rates vary with soil conditions, biological characteristics of the crops, and available supply of manure or other organic fertilizers. The approximate rates of nitrogenous fertilizers (in kg per ha converted into nitrogen) are 40–60 for winter grains sown after an occupied fallow and 30–40 after a clean fallow; 40–60 for spring grains; 60–120 for silage [[corn]] and for [[grain]] in the nonchernozem zone and northern part of the forest-steppe zone, 45–60 on rich chernozems of the forest steppe, and 120–150 in irrigated regions; 45–60 for sugar beets on chernozems of the forest steppe, 80–120 on gray forest soils, podzolized chernozems of the forest steppe, and in the nonchernozem zone, and 100–150 in irrigated regions; 120–140 for [[cotton]]; 40–60 for fiber [[flax]]; 45–90 for [[hemp]]; 45–90 for [[potatoes]]; 90–120 for [[cabbage]]; 60–90 for [[tomatoes]] and [[cucumbers]]; 60–100 for fruits and berries.<br><br> | ||
| + | Smaller amounts are applied to soils richer in natural nitrogen or when manure or other nitrogen-containing organic fertilizers are used at the same time. If nitrogenous fertilizers are in ample supply, the rates can be increased in humid regions; this generally increases the yield and improves the quality of the crops. For example, good nitrogen nutrition favors the formation of gluten in [[wheat]] [[grain]] and increases the protein content of fodder crops.<br><br> | ||
| + | Nitrogenous fertilizers are used as the base fertilizer and as supplementary feeding. They are added to winter crops which were sown after a clean fallow only as early spring supplements (30–40 kg of nitrogen per ha) on semithawed soil (on the ground’s ice “crust”). <br><br> | ||
| + | See also Fertilizer (or Fertiliser)<br><br> | ||
| + | ==Shipment / Storage / Risk factors== | ||
| + | Over the last few years various problems have been encountered, associated with coagulation and compaction of prilled nitrogenous fertilizer in bulk. The majority of fertilizer carried is in the form of urea, [[Ammonium Nitrate]] or sodium/[[Potassium Nitrate]]. The fertilizer can be shipped in 50 kg bags, in flexible intermediate bulk containers (FIBC) up to one tonne or in bulk for bagging on completion of discharge. <br><br> | ||
| + | On arrival at the discharge ports it is not uncommon for the prilled fertilizer to be found in a compacted state. This condition may easily be identified if the product is in pure bulk form; however, it may not so easily be identified if carried in [[Bulk Bags]] or palletised bags. <br><br> | ||
| + | <b>Compaction or coagulation may occur prior to discharge due to one or more of the following reasons:</b><br> | ||
| + | |||
| + | 1. Insufficient drying carried out prior to loading. If the product is dried utilizing a blown air system there should be sufficient volume of air to carry out the drying process.<br> | ||
| + | 2. Insufficient anti-caking agent applied. If anti-caking agent is used it is most important that the correct application rate should be applied.<br> | ||
| + | 3. Insufficient priming/curing time prior to loading. Anti-caking agents require time to act before loading or bagging. <br> | ||
| + | 4. Loading/bagging in conditions of high humidity. During times of high humidity many prilled fertilizers become remarkably hygroscopic and will absorb moisture from the atmosphere.<br> | ||
| + | 5. Water ingress during sea passage. This may occur either due to leaking hatches or ingress via the bilge system.<br> | ||
| + | 6. Moisture due to condensation. If the passage is subject to variable temperatures, condensation may form on the ship’s structure and fall on to the cargo. Ventilation on passage may have to be considered but instructions should be sought from shippers.<br> | ||
| + | 7. Natural compaction due to motion of the vessel whilst on passage.<br><br> | ||
| + | <b>The problems encountered by the farmers as end users may be listed as follows:</b><br> | ||
| + | 1. Time factor. The time taken to break down the lumps manually to a free flowing state may greatly increase the time taken to spread a given acreage. <br> | ||
| + | 2. Uneven spread rate. If the lumps are not completely broken down, blocking of the spreader outlet may occur, reducing the application rate or even cutting off the flow completely. This results in uneven spread of the product. Crop reduction may be a direct result. <br> | ||
| + | 3. Spillage. A considerable amount of spillage may occur when large lumps drop into free flowing prills. This has an effect of increasing time taken and also wastage. <br> | ||
| + | 4. Damage to [[machinery]]. The unexpected dropping of large lumps of product into hoppers may result in damage being occasioned to the hopper sides or distortion of the filter grid.<br> | ||
| + | 5. Safety implications. Various comments have been made to surveyors concerning this aspect. Where bags are suspended over the spreader hopper and the bottom of the bag slit to allow product to flow into the hopper, some party of the human body will of necessity be partially below the bag during this operation. If the bag is slit and the weight of the solid lump splits the bag further and suddenly drops, injury may result. <br><br> | ||
| + | It should be pointed out that the compaction or coagulation does not impair the quality of the product once it has been broken down to a usable free-flowing condition. However, this saving is not sufficient to compensate farmers for the additional time taken to break down the lumps to a useable condition. Late deliveries may aggravate the problems, forcing the farmers to accept the product rather than reject it and await fresh delivery. | ||
| + | |||
| + | |||
| + | [[Category: Products]][[Category: Seeds and agriproducts]] | ||
| + | |||
[[Category:Seeds and agriproducts]] | [[Category:Seeds and agriproducts]] | ||
[[Category:Products]] | [[Category:Products]] | ||
Latest revision as of 15:18, 29 January 2014
| Infobox on Nitrogenous Fertiliser | |
|---|---|
| Example of Nitrogenous Fertiliser |  |
| Facts | |
| Origin | - |
| Stowage factor (in m3/t) | 1,15 m3/t (in bulk) |
| Humidity / moisture | - |
| Ventilation | - |
| Risk factors | See text |
Nitrogenous Fertiliser
Description
Nitrogenous fertilisers are mineral and organic substances used as a source of nitrogen nutrition for plants. They are divided into organic fertilizers (manure, peat, compost) containing other nutrients besides nitrogen; manufactured mineral fertilizers; and green fertilizers (lupine, serradella).
Ammonia fertilizers include ammonium sulfate, ammonium chloride, ammonium bicarbonate, and liquid nitrogenous fertilizers. Ammonium sulfate and ammonium chloride are most effective on base-saturated soils (chernozems, calcareous serozems, chestnut) which can neutralize their acidifying effect. Systematic fertilization with ammonium sulfate or ammonium chloride increases soil acidity, which can be corrected by liming. Ammonia nitrogen is less susceptible to leaching than nitrate nitrogen; thus, ammonia fertilizers can be added in the fall, before planting. They are less suitable for surface (as supplementary feeding for winter crops) and local (in rows or by cluster sowing) application. Excessive chlorine in ammonium chloride has an adverse effect on the size and quality of the yield of many crops, including potatoes, flax, oil-producing plants, tobacco, and grapes. Ammonium bicarbonate, still produced only in limited quantities for experimental purposes, has an alkaline reaction, but it undergoes nitrification in soil. Among the ammonia forms, liquid fertilizers—liquid anhydrous ammonia, aqua ammonia, and ammoniates—are the most important.
Nitrate fertilizers include sodium nitrate (Chilean saltpeter), calcium nitrate (lime saltpeter, Norwegian saltpeter), and Potassium Nitrate. Sodium nitrate is physiologically alkaline and is therefore best applied to acid soils, especially when sugar beets, wheat, barley, and other acid-sensitive crops are grown. Calcium nitrate is put up in pellets usually admixed with Ammonium Nitrate; it, too, alkalizes the soil. Potassium nitrate contains potassium as well as nitrogen and is a source of nitrogen-potassium nutrition for plants. It is applied to chlorine-sensitive crops. All the nitrate forms of nitrogen are not absorbed by the soil. In regions with excess moisture, nitrate fertilizers are leached out of light soils with weak water-retention capacity. It is best, therefore, to use ammonia fertilizers as the main fertilizers.
Amide fertilizers include urea (carbamide), calcium cyanamide, and urea-formaldehyde. Urea is the most valuable. In soil it readily changes into ammonium carbonate; it first slightly alkalizes the soil and then weakly acidifies it. It is recommended that urea be added early. It is also used as a protein supplement for ruminants. Calcium cyanamide is able to reduce soil acidity.
Properties of the main mineral nitrogenous fertilizers
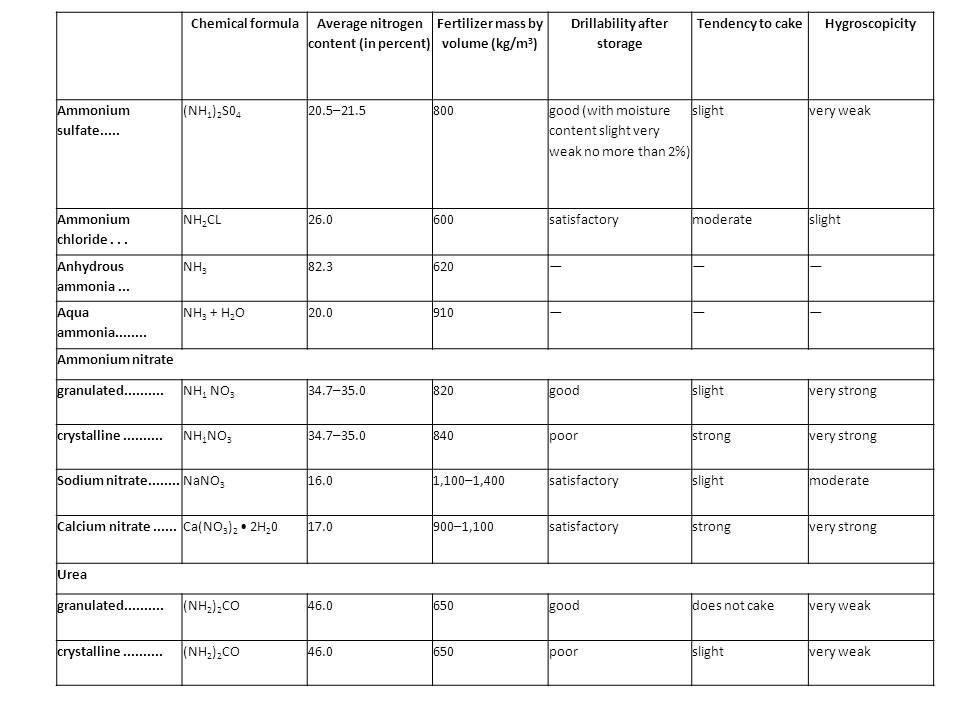
If applied in the fall, it is effective on mellow neutral soils rich in organic matter. It is unsuitable for local application. Calcium cyanamide is also used as a defoliant to remove leaves from cotton before it is harvested. Urea-formaldehyde fertilizers are not leached out of the soil and are especially effective in regions of excess moisture and irrigated agriculture. The use of large amounts provide crops with enough nitrogen to last several years. The characteristics of mineral nitrogenous fertilizers are given in the table.
Nitrogenous fertilizers are an effective means of increasing crop productivity, especially in the nonchernozem zone, humid regions of the forest steppe, and the zone of irrigated agriculture where the soils do not contain enough nitrogen. The fertilization rates vary with soil conditions, biological characteristics of the crops, and available supply of manure or other organic fertilizers. The approximate rates of nitrogenous fertilizers (in kg per ha converted into nitrogen) are 40–60 for winter grains sown after an occupied fallow and 30–40 after a clean fallow; 40–60 for spring grains; 60–120 for silage corn and for grain in the nonchernozem zone and northern part of the forest-steppe zone, 45–60 on rich chernozems of the forest steppe, and 120–150 in irrigated regions; 45–60 for sugar beets on chernozems of the forest steppe, 80–120 on gray forest soils, podzolized chernozems of the forest steppe, and in the nonchernozem zone, and 100–150 in irrigated regions; 120–140 for cotton; 40–60 for fiber flax; 45–90 for hemp; 45–90 for potatoes; 90–120 for cabbage; 60–90 for tomatoes and cucumbers; 60–100 for fruits and berries.
Smaller amounts are applied to soils richer in natural nitrogen or when manure or other nitrogen-containing organic fertilizers are used at the same time. If nitrogenous fertilizers are in ample supply, the rates can be increased in humid regions; this generally increases the yield and improves the quality of the crops. For example, good nitrogen nutrition favors the formation of gluten in wheat grain and increases the protein content of fodder crops.
Nitrogenous fertilizers are used as the base fertilizer and as supplementary feeding. They are added to winter crops which were sown after a clean fallow only as early spring supplements (30–40 kg of nitrogen per ha) on semithawed soil (on the ground’s ice “crust”).
See also Fertilizer (or Fertiliser)
Shipment / Storage / Risk factors
Over the last few years various problems have been encountered, associated with coagulation and compaction of prilled nitrogenous fertilizer in bulk. The majority of fertilizer carried is in the form of urea, Ammonium Nitrate or sodium/Potassium Nitrate. The fertilizer can be shipped in 50 kg bags, in flexible intermediate bulk containers (FIBC) up to one tonne or in bulk for bagging on completion of discharge.
On arrival at the discharge ports it is not uncommon for the prilled fertilizer to be found in a compacted state. This condition may easily be identified if the product is in pure bulk form; however, it may not so easily be identified if carried in Bulk Bags or palletised bags.
Compaction or coagulation may occur prior to discharge due to one or more of the following reasons:
1. Insufficient drying carried out prior to loading. If the product is dried utilizing a blown air system there should be sufficient volume of air to carry out the drying process.
2. Insufficient anti-caking agent applied. If anti-caking agent is used it is most important that the correct application rate should be applied.
3. Insufficient priming/curing time prior to loading. Anti-caking agents require time to act before loading or bagging.
4. Loading/bagging in conditions of high humidity. During times of high humidity many prilled fertilizers become remarkably hygroscopic and will absorb moisture from the atmosphere.
5. Water ingress during sea passage. This may occur either due to leaking hatches or ingress via the bilge system.
6. Moisture due to condensation. If the passage is subject to variable temperatures, condensation may form on the ship’s structure and fall on to the cargo. Ventilation on passage may have to be considered but instructions should be sought from shippers.
7. Natural compaction due to motion of the vessel whilst on passage.
The problems encountered by the farmers as end users may be listed as follows:
1. Time factor. The time taken to break down the lumps manually to a free flowing state may greatly increase the time taken to spread a given acreage.
2. Uneven spread rate. If the lumps are not completely broken down, blocking of the spreader outlet may occur, reducing the application rate or even cutting off the flow completely. This results in uneven spread of the product. Crop reduction may be a direct result.
3. Spillage. A considerable amount of spillage may occur when large lumps drop into free flowing prills. This has an effect of increasing time taken and also wastage.
4. Damage to machinery. The unexpected dropping of large lumps of product into hoppers may result in damage being occasioned to the hopper sides or distortion of the filter grid.
5. Safety implications. Various comments have been made to surveyors concerning this aspect. Where bags are suspended over the spreader hopper and the bottom of the bag slit to allow product to flow into the hopper, some party of the human body will of necessity be partially below the bag during this operation. If the bag is slit and the weight of the solid lump splits the bag further and suddenly drops, injury may result.
It should be pointed out that the compaction or coagulation does not impair the quality of the product once it has been broken down to a usable free-flowing condition. However, this saving is not sufficient to compensate farmers for the additional time taken to break down the lumps to a useable condition. Late deliveries may aggravate the problems, forcing the farmers to accept the product rather than reject it and await fresh delivery.











