Difference between revisions of "Bulk Oils and Fats"
(→Tank surface conditions) |
|||
| (12 intermediate revisions by 4 users not shown) | |||
| Line 117: | Line 117: | ||
Zinc silicate coating is an anti corrosive paint system that is based on zinc dust (86% wt) with some additives and a binder. The high levels of zinc dust giving zinc-zinc metal contact resulting in cathodic protection similar to those obtained from galvanising. Zinc coatings have a good resistance against solvents, but are not resistant against strong acids and bases.<br><br> | Zinc silicate coating is an anti corrosive paint system that is based on zinc dust (86% wt) with some additives and a binder. The high levels of zinc dust giving zinc-zinc metal contact resulting in cathodic protection similar to those obtained from galvanising. Zinc coatings have a good resistance against solvents, but are not resistant against strong acids and bases.<br><br> | ||
<b>Cargo tank coated </b><br> | <b>Cargo tank coated </b><br> | ||
| − | Epoxy coatings e.g. pure epoxy, phenolic epoxy and [[isocyanate]] epoxy form cross linkages to different degrees resulting in relatively good resistance to a greater range of cargoes. Epoxy systems are usually resistant to some weak acids and strong alkalies and do not absorb oil-like substances. Epoxy coatings tend to absorb, however, solvent-like cargoes (Resistant with limitations according coating resistance list). This absorption is caused by swelling and subsequent softening of the coating. After transporting aggressive cargoes, the coating tank has to be ventilated until the cargo has been desorbed (released) from the coating film, which results in hardening and decreasing swelling. This can take up to several days, depending on type of cargo, type of coating and film thickness. Water may not be used for cleaning until this ventilation process is finalized. Otherwise the water can | + | Epoxy coatings e.g. pure epoxy, phenolic epoxy and [[isocyanate]] epoxy form cross linkages to different degrees resulting in relatively good resistance to a greater range of cargoes. Epoxy systems are usually resistant to some weak acids and strong alkalies and do not absorb oil-like substances. Epoxy coatings tend to absorb, however, solvent-like cargoes (Resistant with limitations according coating resistance list). This absorption is caused by swelling and subsequent softening of the coating. After transporting aggressive cargoes, the coating tank has to be ventilated until the cargo has been desorbed (released) from the coating film, which results in hardening and decreasing swelling. This can take up to several days, depending on type of cargo, type of coating and film thickness. Water may not be used for cleaning until this ventilation process is finalized. Otherwise the water can lead to blistering and subsequent serious damage of the coating. The more solvency power a cargo has, the more cargo residues could still be present in the coating. This could lead to either contamination of the next or after next cargo or breakdown of the coating film.<br><br> |
See also '''[[Fats and Oils]]'''<br><br> | See also '''[[Fats and Oils]]'''<br><br> | ||
| + | http://www.gard.no/ikbViewer/Content/20651198/Liquid%20bulk%20cargo%20sampling%20-%20collecting%20evidence.pdf<br><br> | ||
| − | http://www. | + | http://www.swedishclub.com/upload/Loss_Prev_Docs/Cargo/Procedures_for_sampling_mineral_oil_cargoes.pdf<br><br> |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category: Products]][[Category: Oil and chemicals]] | [[Category: Products]][[Category: Oil and chemicals]] | ||
Latest revision as of 15:35, 18 January 2021
Contents
Description
Instances of apparent loss by weight or volume may often be accounted for by non-fortuitous causes. Oils and fats in bulk which solidify and require to be heated to liquefy and be pumped out will give a false reading when gauges if the contents of the tank are not completely liquefied. The temperature of the liquid portion, if used in the calculations, will not allow for expansion of the still-solidified portion and the volume indicated will be less than it would be if all were liquid. Quality samples would also be unrepresentative if taken an only partly liquefied bulk.
Too rapid heating may cause burning of the commodity adjacent to the steam coils and shippers’ instructions in this respect should be observed. Unavoidable normal losses by adhering of oil/fat to tank walls, pipelines, etc., are often allowed for by the terms of the sales contracts and a surveyor unfamiliar with bulk oil carriers, tank calibrations, sampling procedures, significance of moisture content, etc., should consult an expert. Where contamination is suspected or confirmed, an analyst conversant with the commodity should be appointed to sample and/or test. The sampling to be carried out, where possible, in the presence of all interested parties.
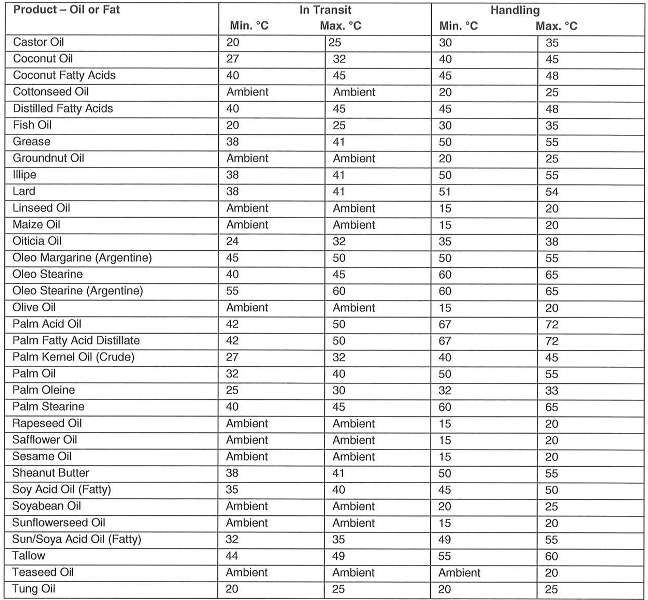
Notes
1. It is recognized that in hot climates ambient may exceed maximum recommended temperatures
2. Although in temperature climates oils or lower viscosities may be stored in tanks without heating – for pumpability and good drainage, a temperature lower than 15°C is not recommended
3. Oils and fats spoil by readily becoming rancid. Rancidity is promoted by light, atmospheric oxygen and moisture and leads to changes in odor and taste. Thus, the tanks must be filled as full as possible, taking into consideration the coefficient of cubic expansion, so that as little ullage space as possible is left above the cargo.
Subject to compliance with the appropriate temperature ranges, duration of storage is not a limiting factor as regards transport and storage life.
If its solidification point is relatively low, the oil does not normally have to be heated. However, if temperatures should arise during the voyage which are in the solidification range, the following must be noted: to be able to pump the oil out of the tanks, it must be at the required pumping temperature. This is only possible, however, if the oil has been kept liquid during the voyage (above a minimum temperature). Loading, travel and pumping temperatures must be precisely complied with, since any change in consistency which occurs during transport may prove irreversible.
If the oil solidifies in the tanks, it cannot be liquefied again even by forced heating. In the vicinity of the heating coils, the oil melts, scorches, discolors and becomes rancid.
Pumping out may be difficult in cold weather. The oil may cool too rapidly in the long lines and solid deposits form on the outer walls, which cannot be pumped out and prevent the still liquid cargo from reaching the suction valve. This problem can be solved by appropriate heating or insulation of the lines.
Deterioration and contamination
Edible oils will suffer quality (essentially flavour) deterioration because of chemical changes within them. In addition they may be contaminated by substances which promote quality deterioration or other adverse chemical changes or which may themselves pose a public health hazard, or simply push the oil out of specification.
Deterioration
Of the adverse chemical changes which may occur during transport and storage oxidation, which is the major cause of rancity, is the most likely. The risk of oxidation increases with the degree of unsaturation and this risk is increased by several factors.
Contact with air.
Tanks must be designed to drain completely so that no pockets of oil remain standing, waiting to be oxidized. For the same reason valves must have no pockets in which oil may lodge and pipelines should drain to a low point fitted with a drain valve and be designed for ‘pigging’. Pumps must not admit air. Lines to tanks, including ship’s tanks, must enter at the top and reach to the bottom to prevent cascading. Air already present in the oil (dissolved oxygen) may be removed, or at least greatly reduced, by sparging with nitrogen, which in effect, sweeps the oxygen out. Aeration of the oil during pumping and tank filling is to be minimized and guidelines for handling oils emphasize this. On the face of it, half or incompletely filled ship’s tanks with the inevitable slopping will promote oxidative rancidity but the addition of antioxidants, as provided for in edible oil standards, and a blanket of inert gas, such as nitrogen, as provided for in modern guidelines greatly reduce the danger.
Light
Light of different wavelengths and at quite low levels for short periods has been shown to promote oxidative flavours, but under the conditions of oil storage and handling it is less important than other factors. The main danger with light is in the transparent bottles used in retail packs and in carelessly stored samples which could give misleading results.
Heat
The rate of chemical reactions generally increases sharply with rising temperatures. The oxidation of oil is no exception but too rapid application of heat may well cause local ‘hot spots’ with concomitant chemical changes quite different from those associated with rancidity. All the literature on the handling of oils emphasized the need for great care in the rate of application of heat and in the temperatures at which oil is pumped. Hot water, thermostatically controlled, should be circulated through stainless steel coils in the tanks.
Metals
Copper, iron, cobalt, nickel and manganese, for example, will catalyse the oxidation of oils. Of these, the first two are of most practical concern. Copper is especially dangerous; its pro-oxidant effect is clearly seen at concentrations as low as 0,02 ppm. Thus, it cannot be over-emphasized that copper must not be present in oil storage and handling systems, either as copper itself or as any of its alloys such as brass, bronze or monel metal. Iron is a less efficient pro-oxidant than copper. Nevertheless its effect can be shown at iron concentrations of 0,5-1,0 ppm. Hence the pick-up of iron from exposed surfaces as rust stains is to be avoided. It is because of pro-oxidant effects of these two metals that Codex Atandards for edible oils set maxima of 0,1 mg/kg (ppm) for copper and 1,5 for iron.
Water
Water promotes hydrolytic rancidity, the splitting of fat to form Fatty Acids. The flavour produced has been described as ‘soapy’ and is different from that resulting from oxidative rancidity, but it is no more pleasant. It is assumed that oil shipped will be waterfree or so low in water as to be free from risk from this source. This assumption is not always justified and tests for water should be routinely carried out on all edible oil samples. It should be obvious that on shipment by sea all hath covers must be watertight.
Microbiological changes
In general, because of the very low moisture content microbiological spoilage of edible oils is not a problem but it is quite possible that with the increasing movement of edible oils strains will develop which will grow on them and a microbiological problem will emerge. This would be favoured by careless cleaning, but in any case if water is present mould growth may occur. It is sometimes associated with Coconut Oil in which an unpleasant odour results and a soapy flavour similar to that resulting from hydrolytic rancidity may also be expected. But it is known with other oils also and fat-splitting moulds increase free fatty acids, again all adverse flavour effects, and reduce bleachability. Good manufacturing practice and good housekeeping in the production of the oil should keep the water content below the very low acceptable limit (0,2%). These and secure closures throughout shipment should protect the oil from mould growth and other forms of microbial deterioration.
Contamination
Contamination begins in the fields and is possible at all stages, right to the consumer’s plate. It is important to recognize the sources of it and to ensure, by sampling and analysis as the oil changes hands, the degree of responsibility at each stage. The sources of contamination are several and some are to be found before the primary products are processed.
Farm and plantation
All agricultural products are likely to contain evidence of the environment from which they come; dirt and dust, fragments of vegetable matter, insect parts, and so on. Other insoluble impurities appearing in crude oils will come as precipitates from the oils themselves. Most of the farm rubbish and the materials from the original plant and from the oil itself will have been removed before the oil is shipped but some may carry over and some extra precipitation from the oil is possible.
Agricultural chemicals
Pesticide residues are the most common and are almost inevitable. Although edible oils are secondary products derived from the processing of crops there will be some carry over of pesticide residues into the oil. Some countries have specific limits for specific pesticides but in any case all pesticides may be covered by the catch-all regulation banning harmful substances.
Hydrocarbons
Some Oil Seeds are dried in the field with hot gases from oil burners. Copra is often dried in ovens or kilns fired by coconut shells. In each case contamination with diesel fuel fumes and/or polyaromatic hydrocarbons is inevitable. The only question is, to what extent? Some hydrocarbons occur naturally in plants and appear in plant products. The concentrations are low and their presence went unnoticed until modern analytical technology revealed it. For the most part there is no database from which deductions of subsequent contamination may be made. Accordingly, tight sampling before and after shipment is essential. Even this may not tell the whole story, however, because reactions may occur within the oils themselves and some of these result in hydrocarbon formation.
Metal pick-up
So long as uncoated mild steel tanks are accepted there is a danger of mechanical contamination by rust flakes or powder or of the formation or iron salts by interaction with fatty acids in acid oils. Accordingly, such tanks are often coated with inert materials (‘plastics for food contact’). If such a coating is discontinuous for any reason (e.g. cracking or pin holing) metal pick up may occur. The dangers of metal contamination can be eliminated altogether by using stainless steel as is normal practice in the rest of the food industry.
Tank coatings
Tank coatings are usually polymeric plastic coatings, mainly polyurethane or epoxy resins. Polyurethane is not included in the BPF Code of Practice and should be avoided in all food applications including the carriage of vegetable and fish oils. Epoxy resins are used in coatings for a variety of products throughout the food industry and are resistant to vegetable oils. Zinc silicate is sometimes used but if in contact with water at higher temperatures fats and oils will hydrolyse to some extent to yield fatty acids which attack it. But Palm Oil and coconut oil should not in any case be carried with this coating. For satisfactory performance the underlying surface must be properly prepared and it is not unreasonable that this should be specified, which calls for the metal to be sand or shot blasted to bright metal before it is coated (Swedish Standard SA3). This standard is accepted as the highest available and produces a ‘white-grey steel with no blemishes’ as a preparation for zinc silicate and epoxy coatings. In all plastics, low concentrations of residual monomers and quite high concentrations of plasticizers may be expected and migration into the food occurs, but the volume of oil relative to the surface coated is high.
Flakes from tank coatings may contaminate oils as a result of mechanical damage, e.g. leaving a Butterworth machine to swing about in an empty tank, or from poor housekeeping. Blisters, which retain residues of a previous cargo, may form in coatings and must be opened, drained, cleaned, and the area resealed. Attention to detail and efficient surveying should enable both ship and shipper to contain contamination from such sources. Tanks should always be inspected before use no matter what the previous cargo was, and loose coating removed and damage made good.
Less easy to detect and deal with is the absorption into a coating of small amounts of a previous cargo. Such contamination may well resist normal cleaning procedures but be released into oils over the course of a voyage. Together with the migration of normal constituents referred to above, this has led to considerable research and development in which government, industry organizations and individual companies have collaborated. Not surprisingly, new coatings, for which claims of greatly improved performance have been made, have been introduced within the last 2 years.
Discharge sequence
While the trend is for shipowners to guarantee total segregation of edible oil cargoes and dedicated pumps and pipelines, common pumps and pipelines are still in use. Where this is so, significant contamination is reduced by strict adherence to the following discharge sequence: edible oils, fully refined, partly refined, crude; technical grade oils; acid oils; fatty acids; other liquid cargoes.
Previous cargoes
The danger of contamination by previous cargoes is well known. It derives from blind pipes, extra valves, careless cleaning, inattention to the clearing and cleaning of all tanks, pipelines, pumps and valves, and damaged or unsuitable coatings with the associated problems referred to above. The remedies are meticulous housekeeping for all types of installations, stainless steel instead of mild steel plus coatings, rigid adherence to the recommendations on cleaning such as the Tank Cleaning Guide of the Chemical Laboratory of ‘Dr. A. Verwey’, Rotterdam, and the Gamlen Tank Cleaning Manual of the Gamlen Chemical Company, and some knowledge of the products previously carried and those about to be loaded. Cleaning will involve hot and cold water washes, sometimes prolonged steaming to ensure the removal of potential taints, rinsing with deionised water, draining and drying.
Of particular relevance, however, is the introduction by the major trade organisations, FOSFA and NIOP, of lists of substances which are acceptable as previous cargoes in tanks which are to be used for the carriage of edible oils (Acceptable Previous Cargo Lists), and of others which are unacceptable (Banned Lists). These lists were developed from late in 1988 and, while always subject to revision, seem to have settled down for the time being.
Reaction products
Edible oils are chemically reactive and the reaction products with hydrogen, oxygen and water are well characterized. There is the possibility therefore that they may well react with other substances leading to the production of taints and even of harmful products. Little is known about this but that the possibility exists lends further emphasis to the need to reduce all contamination to a minimum and to eschew the carriage of some substances in tanks which are subsequently to be used for edible oils.
Methodology
The edible oil industry has been and continues to be active in the development of methods for screening its raw materials for all kinds of contaminants. Several methods have been finalized and it would be unrealistic not to expect future specifications for the shipment of edible oils to be tighter.
Deterioration
The major causes of deterioration are oxidation and hydrolysis, both of which lead to rancidity, possibly the major quality problems in oils. The following are the major tests:
Taste
Crude and part processed oils are not tasted but RBD oils if tasted by a trained palate or taste panel will quickly show whether or not there is incipient rancidity.
Colour
Clean, colourless oils are required by the food industry and most oils are coloured, naturally for the most part, but some darkening is due to oxidation. Whereas taste is subjective, colour may be measured objectively and deterioration against a standard noted.
Tests for Oxidation
Peroxide value(PV)
This measures damage which has occurred by estimating the amount of oxygen in the oil. The PV is the number of multi-equivalents of oxygen per kilogram of oil.
Anisidine value (AnV)
When an oxidized oil is heated in vacuo, as during bleaching and deodorization, its peroxide value falls and its flavour improves, but it is still damaged and may, in fact, oxidize (go rancid) more quickly than an undamaged oil. This test was therefore developed to detect and estimate oxidation breakdown products (carbonyl compounds), i.e. what has happened to the oil as distinct from the PV which describes its present state. It is not a precise method but is a good example of a useful test developed by an industry for its own purposes.
Totox value(total oxidation value). This is a figure arrived at from the two previous tests thus:
Totox value = 2PV + AnV
As a useful guide to oil quality Rossel believes that PV, AnV and totox value should each be below 10.
Kreis test
Like the anisidine test, this test is colorimetric depending in this case on the reaction of phloroglucinol with a number of oxidation and related products to produce a red colour. It is a rapid test indicating rancidity and incipient rancidity.
Thiobarbituric acid (TBA) test
This is an empirical test somewhat similar to AnV but considered to be less useful than PV.
Tests for hydrolysis
Free fatty acids (FFA)
Whereas the above tests yield information on oxidative rancidity, measurements of the FFA is an indication of the FFAs originally present and/or hydrolytic rancidity. It is also an indication of the loss of yield to be expected on refining a crude oil. FFAs are usually quoted as a percentage of the oil and because this requires the use of a specific molecular weight (when several acids will be involved) the value obtained will always be approximate.
Acid value (AV) is related to the FFA value but is measured as the milligrams of Potassium Hydroxide required to neutralize the FFA in 1 gram of oil. FFA value is about half AV. The acid value of an oil may be used as a measure of quality. However, the acid value of the oil must not be too high, as this denotes an excessively high content of free fatty acids, which causes the oil to turn sour. Discoloration may also occur.
Contamination
That edible oils may be contaminated from farm to factory was recognized early in a number of the classical methods of analysis which were developed to detect gross contaminants such as vegetable detritus and dirt. More modern contaminants such as pesticides require more sophisticated analytical methods. What follows indicated briefly the analytical approach of the oil chemist to the more obvious contaminants and discussed in more detail the analytical problems posed by the more modern contaminants associated especially with the tanker trade, viz. mineral oils and chemicals.
Insoluble impurities
These are easy to measure by dissolving the oil in solvent, filtering, and drying and weighing the residue.
Moisture and volatile matter
By far the commonest impurity is water and the method of determining it, by heating one way or another, includes other volatile materials in the result. Moisture is usually linked with impurities in the acronym MANDI – moisture and impurities. Together they give a rough guide to the quality of a shipment.
Dissolved soap
During the processing of oil FFAs are removed with a hot Caustic Soda wash by which they yield soaps which are then washed out. But FFAs may form insoluble soaps also; hard waters will yield calcium and magnesium soaps and mild steel tank surfaces, uncoated or those with scratched or otherwise discontinuous coatings, will give iron soaps. These cannot be washed out and may be present in RBD oils as contaminants. Such, however, are unlikely to become an issue for shippers of oils unless there is pick up of iron from carelessly maintained coated ship’s tanks and pipelines. There are analytical methods for determining dissolved soap but iron pick-up is detectable by atomic absorptiometry or inductively soupled plasma emission spectrometry (ICP).
Smoke, flash and fire points
These tests are a guide to the content of volatile organic materials especially FFAs and residual hydrocarbons such as solvent extractants.
Mineral oil (hydrocarbons)
Mineral oil (hydrocarbons) will show up in the unsaponifiable material and will affect the smoke, flash and fire points. It may be detected sometimes by fluorescence under ultra-violet light and/or by thin layer chromatography (TLC). Because of the blanket prohibition of the presence of mineral oil in food products in the UK and because of the possibilities of the contamination of edible oils by mineral oil and refined petroleum products from previous cargoes, the detection and measurement of mineral oil hydrocarbons has become of major importance to all concerned with the shipment of edible oils.
IUPAC has a standard method (IUPAC Method 2.611) for the Detection of Mineral Oils in Vegetable and Animal Oils and Fats. It is based on thin layer chromatography and has a detection limit of about 0,01% of ‘saturated hydrocarbons’. A rider to the method states that contamination of the sample with mineral oil is only considered to be positive if more than 0,5% (m/m), i.e. 500 mg/kg (or ppm) of mineral oil is found. This is equivalent to half a tonne of mineral oil in a 1000 MT tank of edible oil and constitutes gross contamination compared with ‘trace’ scenarios of GC. A further note to the method says that ‘under the conditions of the above procedure 0,02% (m/m) of paraffin oil is detectable if 2 mg of sample is used’ and that ‘this amount is of the same order as the natural saturated hydrocarbon content of oils and fats, which is about 0,01 to 0,03% m/m’. It is not a sensitive method, nor can it differentiate between substances present naturally and the not inconsiderable contamination mentioned above. Further, this method is said to be unsuitable for petrol or diesel oil because of the volatility of these hydrocarbon mixtures with consequent loss of at least part of the sample from the chromatographic plates during the analysis. If the contamination is gross it does, however, provide a base from which prosecution of claims for compensation relating to mineral oil contamination may proceed.
Because of the volatility of petroleum and diesel oil fractions, headspace analysis by GC/MS of allegedly contaminated oils is the preferred method, but this method, because of its sensitivity, will possibly detect traces of residual ‘commercial hexane’ used in the extraction of the oil and low carbon compounds formed in the oil by incipient rancidity. The correct interpretation of the results obtained is therefore crucial. Nevertheless, GC/MS is the method of choice for these and other trace substances. As noted earlier, GC is in common use throughout the edible oil trade for plotting the constituent fatty acids of the various oils from which the methodology has been established. Agreed procedures for detecting and measuring hydrocarbons are not difficult to establish and it cannot be emphasized too strongly that GC/MS is capable of detecting these and other substances at the nanogram (parts per thousand million, i.e. 10-9) level. The interpretation of such results, and of those at the higher concentrations of parts per million and above, poses problems because for the most part the database against which they must be judged does not exist.
It is known that long chain hydrocarbons occur naturally on some seed coatings a lot of work needs to be done quickly to establish the range of such compounds, and the range of concentrations in which they may be found, in the major edible oils of commerce, especially (from the shippers’ point of view) in soya bean oil and palm oil products.
It is known too, that changes in oil composition may occur in transit. There may be loss of volatile substances, precipitation from the oil of gummy materials and absorption by these gums of other substances present. Of these the first is likely to relate to hydrocarbons, but all three emphasize the importance of adequate sampling on loading and discharging and of careful analysis of the samples.
Polyaromatic hydrocarbons (PAHs)
By and large these substances are carcinogens. They have no place in any food product. Apart from a few related compounds in the tiniest concentrations, they are not found naturally in edible oils nor are they likely to appear in them during handling or storage, except by contamination by previous cargoes. They may, however, get into edible oils from flue gases during the drying of copra and/or Oil Seeds. Their detection and measurement by GC/MS is straightforward and the shippers’ protection is once again efficient sampling before and after shipment.
Previous cargoes
Apart from mineral oils, i.e. hydrocarbons, in various forms, previous cargoes will include a whole range of chemical all of which can be detected and measured in very low concentrations by GC/MS.
Trace metals
The classical methods of measuring traces of metals were colorimetric and not very sensitive. This changed altogether with the invention in 1957 of atomic absorptiometry. When sodium salts, for example, are volatilized in a flame, the flame turns yellow i.e. the transmitted light is yellow and the intensity of it may be positioned specifically in the spectrum of visible light so that the transmission spectrum of sodium is specific to that element. If only the yellow light is transmitted it follows that all other light is absorbed and it was found that for most metals the absorption spectrum was more useful for analytical purposes than the transmission spectrum. This formed the basis of the development of atomic absorptiometry, an analytical method of great power and elegance which permits the detection and measurement of concentrations of trace metals far lower than heretofore. This method is available for the measurement of metal contamination in edible oils. Its more modern development, inductively coupled plasma emission spectrometry (ICP), allows many trace elements to be detected simultaneously.
Contaminating oils
There are three methods available for testing for the presence of certain other oils. The Halphen test detects low levels of crude cottonseed, kapok and related oils. It depends on the presence in these oils of specific unusual fatty acids but does not apply to processed oils. It is sensitive to as little as 0,1-0,2% of cottonseed oil.
The Baudoin test will identify sesame seed oil by detecting specific compounds in it. It works with processed oils and reveals the presence of 0,5% of sesame seed oil. The Evers test for groundnut oil depends on the fractional crystallization of fatty acids of higher molecular weight and must for this very reason be treated with some scepticism though it is claimed that 5-10% of groundnut oil may be detected in oil mixtures. These tests may, of course, be used to confirm the identity of the particular oils to which they apply.
Micro-organisms
Microbiological contamination is not a major issue in this context, especially if normal precautions are taken. Standard microbiological procedures exist for the detection and identification of moulds and other contaminating micro-organisms and are applied as necessary.
Conformity
There is, perhaps, only one thing to be added to what has gone before. That is that an oil shall conform with the buying specifications of the purchaser and/or the food regulations of the country of destination. It is apparent that there is a wide range of oil-specific and classical industry-accepted tests available for identifying an oil, measuring its quality and examining it for contamination. It is also easy to detect and measure substances such as the antioxidants which give it a measure of protection from oxidation.
There is also available a group of powerful modern analytical methods the application of which to oils greatly facilitates the identification of an oil and the detection of adulterants in it. These same methods have also presented the edible oil industry with powerful tools in its attempts to raise standards in the storage and handling of its products and raw materials but detectable concentrations are so low that once a chemist is alerted to what may be present, it is highly likely that he will detect it. To this extent the disclosure by the ship’s master of the three previous cargoes carried in a tank about to be used for the carriage of an oil will amount to self incrimination in that an analyst competent in the techniques will almost certainly be able to detect a minute trace of what he knows to look for. In this, and in the power of modern analytical technology generally, lie problems of claim, counter-claim and litigation. This same power, has, however, provided the industry with the means of protecting its various constituent parts, of which the shippers are one and those who protect them another, against false claims of contamination provided (1) a sufficient database of background information against which comparisons may be made is built up, (2) sampling is tightly and efficiently carried out every time a parcel of oil is moved, and (3) the industry eschews NIL tolerances and comes to some general agreement on concentrations of contaminants below which oils are acceptable.
Epilogue
Vegetable oils are foods and are thus subject to food regulations especially in the country of destination. Food regulations are concerned primarily with public health and they are being tightened as more knowledge about the incidence of contaminants and their physiological effects accrues, as more effective analytical methodology is introduced, as more consumer action develops and as more media attention is focused on foods generally and especially on bad news about food. The dangers to those who store and ship oils are:
- The threat to public health. This can be real, but in many cases the dilution effect of the large volume of oil reduces the concentration of contamination below any danger level.
- The perceived threat to public health. This is two-fold; one is the US Delaney Clause which forbids the presence, i.e. at any concentration at all, in any food of any substance which has been shown to cause cancer in man or animals regardless of dose or conditions or methods of administration; the other is the sensational press which, in matters of this nature, so easily makes mountains out of molehills.
- The detection by modern sensitive analysis of contamination which opens the way to insurance claims and litigation.
Before the Second World War, food processing equipment was made largely of copper, tinned copper and mild steel. From 1950 onwards, stainless steel has steadily replaced all the other metals, plastics have been introduced, and the scientific and specialized food science literature has shown an increasing emphasis on the control of micro-organisms and the reduction of chemical contamination in the total food chain. Good manufacturing practice (GMP), which includes the handling of raw materials and their protection from all kinds of maltreatment and contamination, calls for the use of stainless steel for food storage and processing and for the careful design of equipment, including pumps and pipelines, to facilitate drainage and to ensure that rigorous cleaning may be carried out. This last is essential if one is to avoid contamination by micro-organisms, chemicals or other food products.
There seems little doubt that in the past, edible oils have been abused during the journey from harvest to consumer. Indeed, the criteria for the shipment of some chemicals have been tougher, for very good chemical reasons, than those applied to edible oils. This has been due in large part to unfamiliarity with requirements for food products and food raw materials and is changing as the industry demands food standard treatment for food products. The ideal is dedicated stainless steel tanks; the practicality is considerably less; but the reality is likely to be:
- Tighter specifications for the plastic coatings acceptable on mild steel tanks, not only relating to composition but also to application and standards of acceptability to industry-approved surveyors.
- A demand for more rigorous draining and cleaning of pumps and pipelines.
- Closer attention to sampling and analysis of samples before and after shipment.
Finally, in the light of the ability of the analytical chemist to detect and measure substances in very low concentrations and below those of public health significance, there is need for the establishment of a database of hydrocarbons naturally occurring in oils and for agreement, at least within the industry, on acceptable levels for a whole range of contaminants.
Tank surface conditions
Cargo tank stainless steel
Stainless steel can corrode in service if there is contamination of the surface. Both pickling and passivation are chemical treatments applied to the surface of stainless steel to remove contaminants and assist the formation of a continuous passive chromium oxide, film. Pickling and passivation are both acid treatments and neither will remove grease or oil. If the steel is dirty, it may be necessary to use a detergent or alkaline cleaning before pickling or passivation.
Zinc silicate coating
Zinc silicate coating is an anti corrosive paint system that is based on zinc dust (86% wt) with some additives and a binder. The high levels of zinc dust giving zinc-zinc metal contact resulting in cathodic protection similar to those obtained from galvanising. Zinc coatings have a good resistance against solvents, but are not resistant against strong acids and bases.
Cargo tank coated
Epoxy coatings e.g. pure epoxy, phenolic epoxy and isocyanate epoxy form cross linkages to different degrees resulting in relatively good resistance to a greater range of cargoes. Epoxy systems are usually resistant to some weak acids and strong alkalies and do not absorb oil-like substances. Epoxy coatings tend to absorb, however, solvent-like cargoes (Resistant with limitations according coating resistance list). This absorption is caused by swelling and subsequent softening of the coating. After transporting aggressive cargoes, the coating tank has to be ventilated until the cargo has been desorbed (released) from the coating film, which results in hardening and decreasing swelling. This can take up to several days, depending on type of cargo, type of coating and film thickness. Water may not be used for cleaning until this ventilation process is finalized. Otherwise the water can lead to blistering and subsequent serious damage of the coating. The more solvency power a cargo has, the more cargo residues could still be present in the coating. This could lead to either contamination of the next or after next cargo or breakdown of the coating film.
See also Fats and Oils
http://www.gard.no/ikbViewer/Content/20651198/Liquid%20bulk%20cargo%20sampling%20-%20collecting%20evidence.pdf











